Table of Contents:
Introduction to Steel Making Converter Process
Alright, let's dive right into the world of steel making, shall we? The converter process is like the heart of modern steel production. It's where raw materials, such as pig iron and scrap steel, are transformed into something incredibly valuable: steel. Imagine it as a grand alchemy, turning base metals into the backbone of our infrastructure.
Now, why is this process so important? Well, it's because about 70% of the world's steel is produced using various oxygen converter methods. This isn't just some small-scale operation; it's a global powerhouse of transformation. The magic happens when oxygen is blown into the molten iron, removing impurities and refining the metal into high-quality steel.
And here's the kicker: this process isn't just about making steel. It's about doing it efficiently and sustainably. The converter process is designed to maximize output while minimizing waste and energy consumption. So, it's not just about what we make, but how we make it. That's the beauty of the steel making converter process. It's a blend of science, art, and a bit of magic, all rolled into one.
Historical Development of Converter Steelmaking
Let's take a little stroll down memory lane, shall we? The journey of converter steelmaking is a fascinating tale of innovation and evolution. It all kicked off in the mid-19th century, when the industrial world was buzzing with new ideas. Back then, the process involved blowing air from the bottom of the converter to oxidize impurities. It was revolutionary for its time, even if it was a bit rough around the edges.
Fast forward to the 1950s, and things really started to heat up. This was the era when oxygen lances were introduced, transforming the process entirely. By injecting pure oxygen from above, the efficiency and quality of steel production skyrocketed. It was like switching from a horse-drawn carriage to a jet plane — a real game changer.
As we approached the end of the 20th century, the technology saw further refinement. Engineers began to combine top and bottom blowing techniques, which improved control and efficiency even more. It was like adding a turbocharger to an already powerful engine. This continuous innovation has made converter steelmaking a cornerstone of modern industry, adapting and evolving to meet the ever-growing demands of the world.
Key Components of the Converter Process
Alright, let's get into the nuts and bolts of the converter process. It's not just about blowing oxygen into molten iron; there's a whole orchestra of components working in harmony to make it all happen. So, what are these key players?
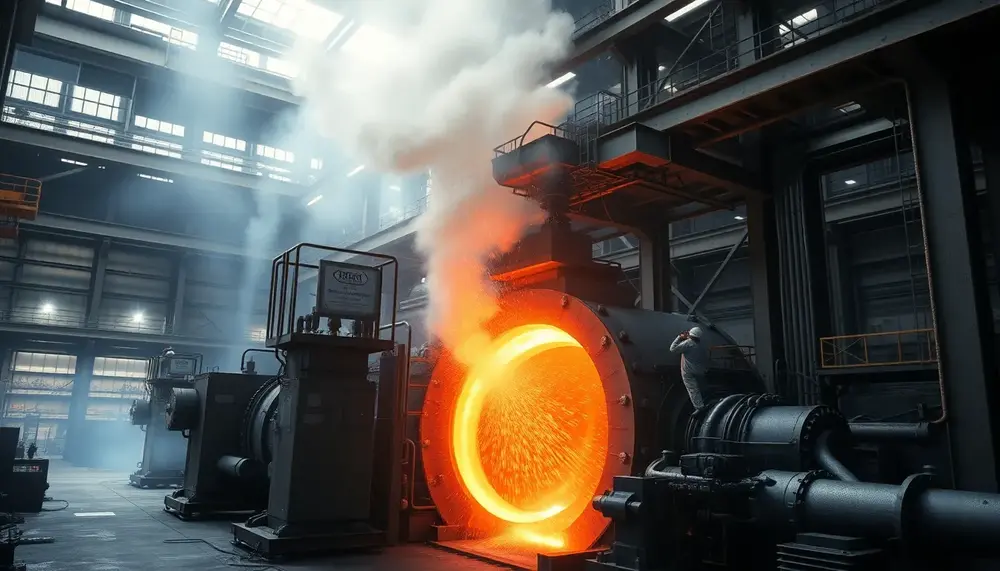
First up, we have the converter itself. This is the big, sturdy vessel where the magic happens. It's designed to withstand extreme temperatures and the intense reactions taking place inside. Think of it as the stage where the steelmaking performance unfolds.
Next, there's the oxygen lance. This tool is crucial for injecting oxygen into the molten metal. It’s like the conductor's baton, directing the flow of oxygen to ensure the impurities are oxidized efficiently. The precision of this component is vital for the quality of the final product.
Then, we have the refractory lining. This is the unsung hero of the process, protecting the converter from the intense heat and chemical reactions. It's made from heat-resistant materials that ensure the converter can keep performing without a hitch.
And let's not forget the slag. This byproduct of the process plays a key role in removing impurities. It floats on top of the molten metal, capturing unwanted elements and ensuring the steel remains pure and strong.
Each of these components is essential, working together like a well-oiled machine to transform raw materials into high-quality steel. It's a complex dance of chemistry and engineering, all aimed at producing the best steel possible.
Chemical Reactions in Steel Converter
Alright, let's dive into the bubbling cauldron of chemistry that is the steel converter. The process is a bit like a high-stakes science experiment, where precise chemical reactions are the name of the game. So, what exactly is going on inside that fiery vessel?
First off, we have the oxidation of carbon. This is one of the main events, where carbon in the molten iron reacts with the injected oxygen to form carbon monoxide (CO) and carbon dioxide (CO2). This reaction is exothermic, meaning it releases a lot of heat, which is crucial for maintaining the high temperatures needed for the process.
Next, there's the removal of impurities like silicon, manganese, and phosphorus. These elements also react with oxygen to form their respective oxides, which are then absorbed by the slag. It's like a cleansing ritual, purifying the molten metal and ensuring the steel's quality.
Then, we have the interaction between the slag and the metal. The slag acts as a sponge, soaking up the unwanted oxides and keeping them separate from the steel. This dynamic relationship is essential for producing clean, high-grade steel.
And let's not forget the role of temperature control. The heat generated by these reactions is carefully managed to ensure the process runs smoothly. It's a delicate balance, like walking a tightrope, where too much or too little heat can affect the final product.
In essence, the steel converter is a chemical symphony, where each reaction plays a vital role in transforming raw materials into something extraordinary. It's a testament to the power of chemistry and engineering working hand in hand.
Specialized Methods for Stainless Steel
Alright, when it comes to stainless steel, things get a bit more specialized. You see, stainless steel isn't just any steel; it's got that shiny, rust-resistant quality that makes it so desirable. But achieving that requires some unique methods in the converter process.
One of the key challenges here is minimizing chromium oxidation. Chromium is what gives stainless steel its corrosion-resistant properties, so keeping it intact is crucial. This is where techniques like the AOD (Argon Oxygen Decarburization) process come into play. By diluting the oxygen with inert gases like argon, the oxidation of chromium is significantly reduced. It's like giving chromium a protective shield against the harsh environment inside the converter.
Another method involves vacuum treatment. By reducing the pressure inside the converter, the boiling point of the elements is lowered, allowing for more precise control over the chemical reactions. This helps in maintaining the integrity of chromium and other alloying elements. It's a bit like cooking at high altitude, where everything needs to be adjusted for the conditions.
These specialized methods ensure that the final product retains its stainless qualities, ready to be used in everything from kitchen appliances to architectural wonders. It's a fine example of how a little tweak in the process can lead to a big difference in the outcome.
Enhancing Environmental Sustainability
Alright, let's talk about going green in the world of steelmaking. Enhancing environmental sustainability in the converter process is more than just a trend; it's a necessity. With the growing concern over climate change, the industry is taking big strides to reduce its carbon footprint.
One major focus is on increasing the use of scrap metal and HBI (Hot Briquetted Iron). By recycling more scrap, the need for raw iron ore is reduced, which in turn cuts down on CO2 emissions. It's like giving old steel a new lease on life, and it's a win-win for both the environment and the economy.
Then there's the challenge of energy management. The converter process is energy-intensive, so finding ways to use energy more efficiently is key. This includes innovations like preheating scrap with a scrap preheating lance and utilizing post-combustion lances to capture and reuse energy that would otherwise be lost. It's all about squeezing every bit of energy out of the process.
Of course, there's also the need for infrastructure to support these green initiatives. This means investing in logistics for scrap collection and ensuring quality control to manage trace elements effectively. It's a complex puzzle, but one that's essential for a sustainable future.
In short, enhancing environmental sustainability in steelmaking is about innovation, efficiency, and a commitment to doing better by our planet. It's a journey, but one that's well worth taking.
Conclusion
So, there you have it. The steel making converter process is a marvel of modern engineering, blending raw materials into something indispensable. From its historical roots to its cutting-edge advancements, this process is a testament to human ingenuity and our relentless pursuit of progress.
We've explored the intricate dance of chemical reactions, the specialized methods for crafting stainless steel, and the ongoing efforts to make the process more environmentally friendly. Each aspect is a piece of a larger puzzle, working together to produce the steel that builds our world.
As we look to the future, the focus will undoubtedly remain on innovation and sustainability. The challenges are significant, but so are the opportunities. With continued dedication and creativity, the steel industry can continue to evolve, meeting the demands of tomorrow while respecting the needs of our planet.
In essence, the steel making converter process is more than just a method; it's a symbol of transformation and resilience. And in a world that's constantly changing, that's something we can all appreciate.
FAQ on Steel Making Using the Converter Process
What is the significance of the steel making converter process?
The converter process is crucial for modern steel production, transforming raw materials like pig iron and scrap steel into high-quality steel and accounting for about 70% of global steel production using various oxygen converter methods.
How did the converter steelmaking process evolve over time?
The process started in the mid-19th century with air oxidation techniques, significantly evolved in the 1950s with the introduction of oxygen lances, and was further refined by combining top and bottom blowing techniques towards the end of the 20th century.
What are the key components involved in the converter process?
Key components include the converter vessel, oxygen lance, refractory lining, and slag, each playing a vital role in ensuring efficient and effective steel production.
What chemical reactions occur in the steel converter process?
The process involves carbon oxidation to CO and CO2, removal of impurities like silicon, manganese, and phosphorus through oxidation, and the interaction of slag with metal for impurity absorption.
How is environmental sustainability enhanced in steelmaking?
Environmental sustainability is enhanced by increasing the use of scrap and HBI, employing energy-efficient technologies such as scrap preheating and post-combustion lances, and improving infrastructure for scrap logistics and quality control.